Print-Friendly PDF | Large-Print PDF
This publication is intended for health care professionals, hospital/clinic staff who are responsible for specifying or purchasing diagnostic medical equipment, medical equipment specialists, and all those who require knowledge of the technical specifications for accessible examination tables and chairs. In order to understand the information provided in this document, a general knowledge of basic terminology and technical concepts used in accessibility standards is desirable, but not necessary. The goal is to provide information about the physical configuration and operational characteristics of accessible examination tables and chairs as specified in the latest proposed federal standards so that medical facilities are properly equipped to serve individuals with disabilities.
I. Introduction: Best Practice versus Regulation
The “Patient Protection and Affordable Care Act” (ACA) added an amendment to Section 510 of the Rehabilitation Act which authorized the U.S. Access Board to develop accessibility standards for medical diagnostic equipment (MDE) in consultation with the Food and Drug Administration. The standards address independent access to, and use of, equipment by people with disabilities to the maximum extent possible. Section 510 describes "medical diagnostic equipment" as equipment that is “used by health care professionals in medical settings, such as physician's offices, clinics, emergency rooms, and hospitals, for diagnostic purposes.” The proposed standards for MDE apply to equipment that includes examination tables, examination chairs (including chairs used for eye examinations or procedures, and dental examinations or procedures), weight scales, mammography equipment, x-ray machines, and other radiological equipment commonly used for diagnostic purposes by health professionals.
Section 510 does not authorize the Access Board to issue scoping provisions for MDE which specify the minimum number of accessible features to be provided in facilities. Also, it does not address who is required to follow the regulatory standards. The proposed standards establish minimum technical criteria that will allow patients with disabilities independent entry to, use of, and exit from medical diagnostic equipment to the maximum extent possible. For example, sections M301 and M302 of the proposed standards address design and operational features that will allow a patient with a disability to independently transfer onto examination chairs and tables used for diagnostic purposes. These proposed standards are not yet enforceable as federal regulations. The proposed standards provide “best practice” guidance for specifying and acquiring accessible MDE. Refer to the text of the U.S. Access Board Proposed Standards for Accessible Medical Diagnostic Equipment (see Resources).
The proposed standards are based on the building blocks and other technical criteria for accessible elements found in the 2004 ADA and ABA Accessibility Guidelines. Other sources of information used in the development of the proposed standards include chapter 16 “Accessibility Considerations” of ANSI/AAMI HE75 Human factors engineering – Design of medical devices, anthropometric data and other standards which address such features as the width of transfer surfaces on diagnostic equipment used by patients in a seated position.
II. Technical Criteria
Chapter M3 of the Proposed Standards provides technical criteria for accessible diagnostic equipment based on the patient positions that the equipment is designed to support, including equipment used by patients in a supine, prone, or side-lying position (section M301); equipment used by patients in a seated position (section M302); equipment used by patients seated in a wheelchair (section M303); and equipment used by patients in a standing position (section M304). Chapter M3 also include technical criteria for equipment supports (section M305), for instructions or other information communicated to patients through the equipment (section M306), and for operable parts used by patients (section M307).
III. Examination Tables and Chairs
Examination tables and chairs are used almost universally throughout the health care delivery system. Therefore, tables and chairs must support a wide range of diagnostic activities, clinical indications, and patient populations. These demands have implications for the design, configuration, and principles of operation of examination tables and chairs. Manufacturers generally design examination tables based on the diagnostic needs and convenience of the medical professionals, with the primary functions of these tables being to support patients in prone, supine or side-lying positions. The exam tables used in most doctors’ offices are typically designed to be used at a fixed height of 32 inches making independent transfer very difficult or impossible for many people with mobility disabilities, especially those whose use mobility aids such as a wheelchair. See the figure below. Manufacturers design examination chairs to meet the primary functions of supporting patients in a seated or “semi-supine” position, as well as the diagnostic needs of the medical professional, but often these chairs do not allow independent transfer for patients with mobility disabilities.

Equipment Features Needed for Patient Support in Supine, Prone, or Side-Lying (M301) or
Seated (M302) Position with Examples of Equipment Types
|
Patient Positions Equipment Designed to Support |
Equipment Features Addressed by the Technical Criteria M301 or M302 |
Examples of Types of Equipment |
|
M301 - Diagnostic Equipment Used by Patients in Supine, Prone, or Side-Lying Position |
· Transfer surface, including height, size, and transfer sides · Transfer supports, stirrups, and head and back support · Lift compatibility |
· Examination tables · Examination chairs designed to recline and be used as examination tables |
|
M302 - Diagnostic Equipment Used by Patients in a Seated Position |
· Transfer surface, including height, size, and transfer sides · Transfer supports, armrests, · Lift compatibility |
· Examination chairs · Imaging equipment designed for use with a seat · Weight scales designed for use with a seat |
IV. Transfer Heights
As with transfer surface heights mentioned in the U.S. Access Board’s 2004 ADA and ABA Accessibility Guidelines, the proposed standards call for a transfer surface height range of 17 inches minimum to 19 inches maximum during patient transfer. This distance is measured from the floor level to the top of transfer surface. This requirement is stated in both section M301 (equipment designed to support prone, supine or side-lying positions) and M302 (equipment designed to support a seated position). This is not a single, fixed height but provides a 2 inch range for compliance with the proposed standards. Advisories in both M301 and M302 further clarify this requirement with the statement “The transfer surface is permitted to be positioned outside of the specified height range when not needed to facilitate transfer.”
V. Example Applications of the Technical Criteria for Accessible Diagnostic Equipment
The two following two examples provide a representative list of features for accessible diagnostic equipment required by the technical criteria established by the U.S. Access Board – Proposed Standards referenced in the the Resources section below.
1. Overview of Section M301 - Diagnostic Equipment for Use by a Patient in a Supine, Prone, or Side-Lying Position: Example = Examination Tables
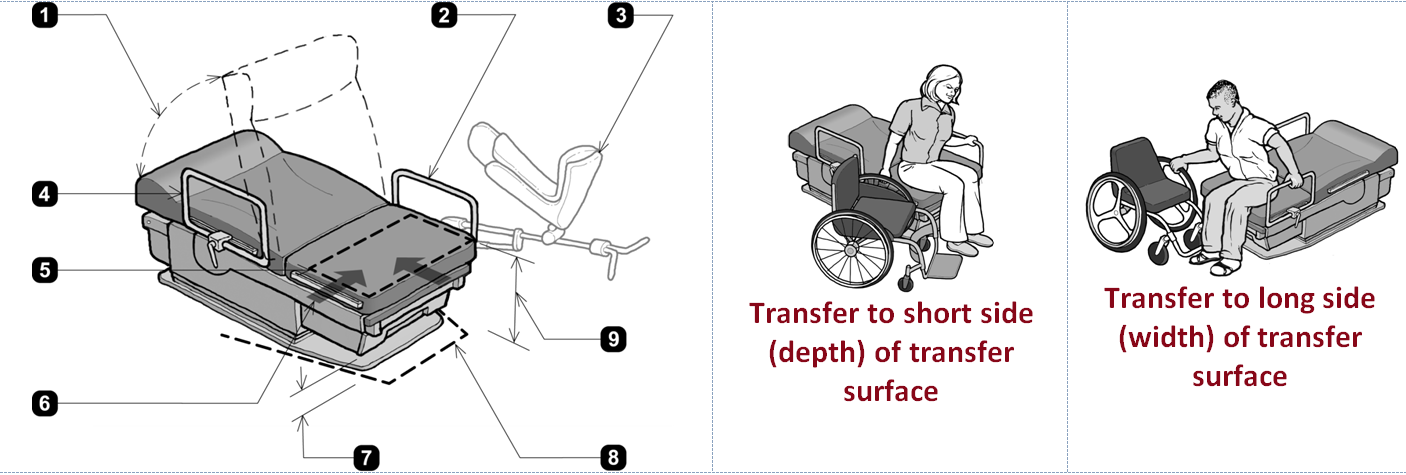
- When adjustable, head and back support provided throughout the entire range of the incline (M301.3.3).
-
Transfer support resists vertical and horizontal forces of 250 lbs at all points and does not rotate within its fittings (M305.2.2 and M305.2.3).
Rail serves as transfer support within reach of transfer surface (M301.3.1 and M305.2.1).
- When provided, stirrups provide a method of supporting, positioning, and securing the patient’s leg (M301.3.2).
- Support rail removable / repositioned to permit unobstructed transfer (M301.2.3 EXCEPTION).
- Transfer surface 30 inches wide minimum and 15 inches deep minimum (M301.2.2).
- One short side (depth) and one long side (width) of the transfer surface permit unobstructed transfer from a mobility device (M301.2.3).
- 6 inches high minimum. Clear above finished floor where equipment overhangs clearance (M301.4.1).
- Base permits clearance around base for a patient portable floor lift, see Figure M2 (M301.4 and M301.4.2
- Transfer surface 17 inches minimum and 19 inches maximum above floor level (M301.2.1), when not needed to facilitate transfer, the transfer surface may be positioned above or below the height range (Advisory M301.2.1).
- Base permits clearance around base for a patient portable floor lift, see Figure M2 (M301.4 and M301.4.2
- Transfer surface 17 inches minimum and 19 inches maximum above floor level (M301.2.1), when not needed to facilitate transfer, the transfer surface may be positioned above or below the height range (Advisory M301.2.1).
2. Overview of M302 Diagnostic Equipment for Use by a Patient in a Seated Position:
Example = Examination Chairs
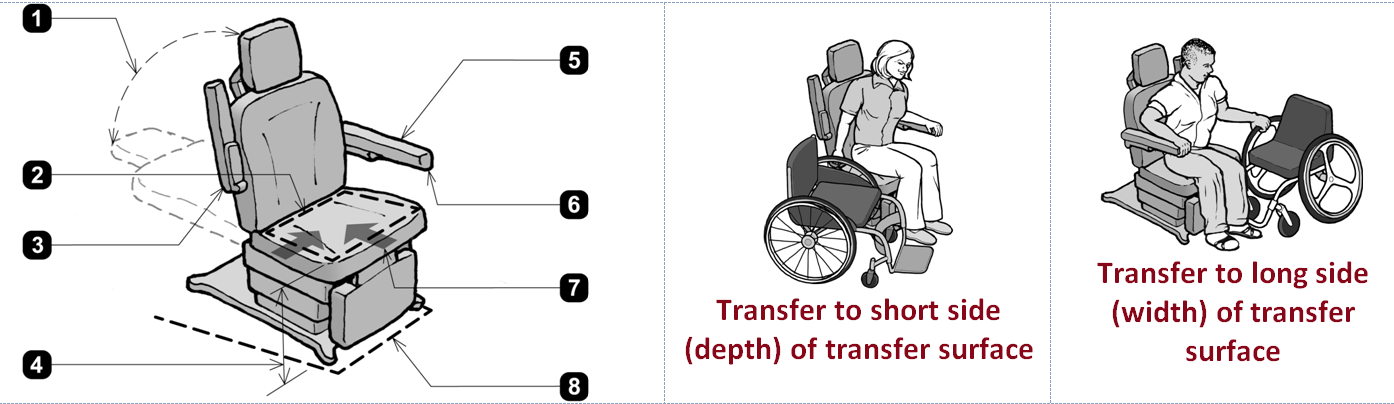
1. When adjustable, head and back support provided throughout the entire range of the incline (M302.3.3).
2. Transfer surface 21 inches wide minimum and 15 inches deep minimum (M302.2.2).
3. Armrest folds up to permit unobstructed transfer (M302.2.3 EXCEPTION).
4. Transfer surface 17 inches minimum and 19 inches maximum. Above floor (M302.2.1), when not needed to facilitate transfer, the transfer surface may be positioned above or below the height range (Advisory M302.2.1).
5. Required armrest serves as transfer support within reach of transfer surface (M302.3.1, M302.3.2, and M305.2.1).
6. Transfer support resists vertical and horizontal forces of 250 lbs at all points and does not rotate within its fittings (M305.2.2 and M305.2.3).
7. One short side (depth) and one long side (width) of the transfer surface permit unobstructed transfer from a mobility device (M302.2.3).
8. Base permits clearance around base for a patient portable floor lift (M302.4 and M302.4.2).
Resources
1. U.S. Access Board – Health Care: http://www.access-board.gov/guidelines-and-standards/health-care
2. Text of the Proposed Standards for Accessible Medical Diagnostic Equipment: http://www.access-board.gov/guidelines-and-standards/health-care/about-this-rulemaking/proposed-standards/text-of-the-proposed-standards
3. Example Applications of Proposed Standards: http://www.access-board.gov/guidelines-and-standards/health-care/about-this-rulemaking/background/example-applications-of-proposed-standards
4. The Barrier Free Healthcare Initiative: http://www.ada.gov/usao-agreements.htm
 The Northwest ADA Center is a member of the ADA National Network. This fact sheet was developed under grant from the Administration for Community Living (ACL), NIDILRR grant #90DP0016-02-00. However, the contents do not necessarily represent the policy of the ACL, and you should not assume endorsement by the federal government.
The Northwest ADA Center is a member of the ADA National Network. This fact sheet was developed under grant from the Administration for Community Living (ACL), NIDILRR grant #90DP0016-02-00. However, the contents do not necessarily represent the policy of the ACL, and you should not assume endorsement by the federal government.
